藤井さんの博士論文がJ Anatomy に受諾されました。
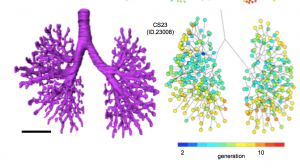
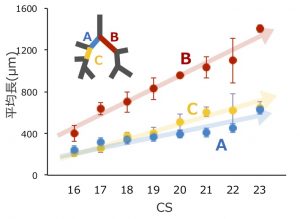
詳細な形態変化情報と正確な分岐次数を算出することにより、これまで明らかでなかった胚子期の気管支形成について新たな知見を与えました。
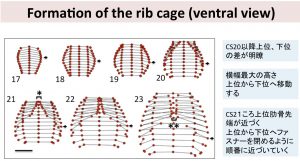
- 胚子期後期までの気管支樹の形態形成をあきらかにした
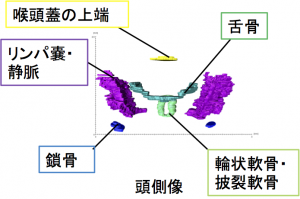
- 気管および大葉気管支の構造は、個人差を示さなかったが、それより末梢では個人差がみられた。
- 右上葉気管支は、右中葉気管支と左上葉気管支の両方の生成後に形成されました。
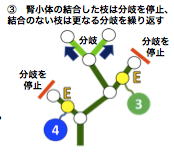
- 右中葉の分岐次数の中央値は、他の 4 葉と比較して有意に低い、
- 右下葉の分岐次数の中央値は、左右の上葉の値よりも有意に大きい。
- CS20 と CS23 の間で見つかったバリエーションはすべて、成人の肺でみられ、気管支樹のバリエーションが胚子期に発生し、生涯続く可能性を示唆
44. Fujii S, Muranaka T, Matsubayash J, Yamada S, Yoneyama A, Takakuwa T. The bronchial tree of the human embryo: an analysis of variations in the bronchial segments. J Anatomy 2020, 237, 311-322. doi: 10.1111/joa.13199.
Abstract
A classical study has revealed the general growth of the bronchial tree and its variations up to Carnegie stage (CS) 19. In the present study, we extended the morphological analysis CS by CS until the end of the embryonic period (CS23). A total of 48 samples between CS15 and CS23 belonging to the Kyoto Collection were used to acquire imaging data by performing phase-contrast X-ray computed tomography. Three-dimensionally reconstructed bronchial trees revealed the timeline of morphogenesis during the embryonic period. Structures of the trachea and lobar bronchus showed no individual difference during the analyzed stages. The right superior lobar bronchus was formed after the generation of both the right middle lobar bronchus and the left superior lobar bronchus. The speed of formation of the segmental bronchi, sub-segmental bronchi, and further generation seemed to vary among individual samples. The distribution of the end-branch generation among five lobes was significantly different. The median branching generation value in the right middle lobe was significantly low compared with that of the other four lobes, whereas that of the right inferior lobe was significantly larger than that of both the right and left superior lobes. Variations found between CS20 and CS23 were all described in the human adult lung, indicating that variation in the bronchial tree may well arise during the embryonic period and continue throughout life. The data provided may contribute to a better understanding of bronchial tree formation during the human embryonic period.