卒業研究発表会(前半)が開催されました(12/27,28)
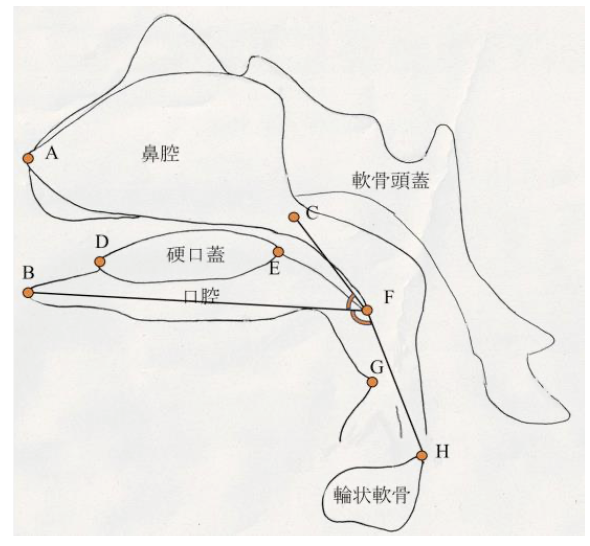
ヒト胎児期初期における咽頭、鼻腔、口腔の形態変化の観察 青江春菜
ヒトの胎児期初期における心室中隔の形態的変化 倭友希
臨床検査技師国家試験を受験する学生は12月いっぱいで卒業研究は終了です。このあとしばらくは国家試験の受験勉強に励んで合格してください。
|
||||
 卒業研究発表会(前半)が開催されました(12/27,28) ヒト胎児期初期における咽頭、鼻腔、口腔の形態変化の観察 青江春菜 ヒトの胎児期初期における心室中隔の形態的変化 倭友希 臨床検査技師国家試験を受験する学生は12月いっぱいで卒業研究は終了です。このあとしばらくは国家試験の受験勉強に励んで合格してください。 26. Formation of tendinous intersections in the human fetal rectus abdominis. MRIを用いたヒト胎児における腱画形成過程の解析 岩佐結生 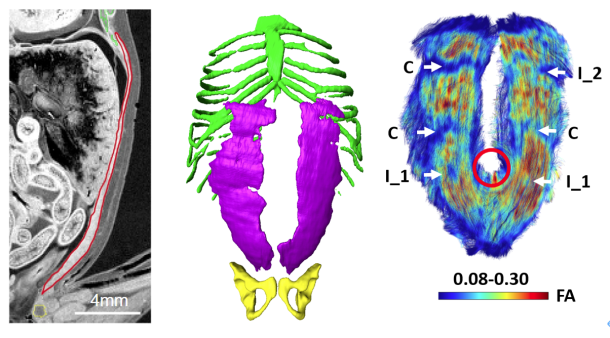 【背景】腱画は腹直筋筋腹を横断する線維性の帯である。これまでは成人を対象に、解剖学および病理組織学的研究によって腱画の形状や位置についての評価がなされてきた。一方でヒト胎児を対象とした研究は少なく、腱画の出現時期や発生的要因については明らかにされていない。本研究では拡散テンソル画像(DTI)を用いて胎児期初期における腱画形成過程を検討することを目的とした。 25. Comparison of Left-Right Differences in Major Blood Vessel Diameter in Human Fetuses. ヒト胎児における主要血管径の左右差の比較検討 中井尚一 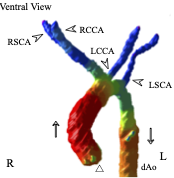 【背景】ヒトの身体は一見左右対称に見えるが、ほとんどの内臓は大きさ、形状、位置の点で左右非対称である。この非対称性は正常な機能のために不可欠である。血管も例外ではなく、ヒト胎児でも大動脈弓や静脈管において定性的な左右差が知られている。一方で血管の左右差について定量的に比較検討した研究はあまり行われておらず、知見も乏しいのが現状である。そこで本研究では、ヒト胎児の主要な血管に着目し、左右差の定性的な知見を深めること及び定量的に比較検討することを目的とした。【対象】京都大学大学院医学研究科附属先天異常標本解析センターが保有するヒト胚子胎児標本24例、島根大学医学部解剖学講座が保有するヒト胎児標本6例、合計30例を高解像度MRIで撮像し、そのMR画像を対象とした (頭殿長: 26.8-225mm) 。標本は正常個体のうち、全身の血管が比較的良好に観察できるものを選択した。検討した血管は頭頸部・上肢の動静脈 (総頸動脈、鎖骨下動脈、腕頭静脈、内頸静脈、鎖骨下静脈) 、肺の動静脈 (肺動脈、上肺静脈、下肺静脈) 、腹部骨盤領域の動静脈 (腎動脈、総腸骨動脈、外腸骨動脈、内腸骨動脈、臍帯動脈、総腸骨静脈) である。【方法】①三次元可視化解析ソフトで対象血管の立体像を5例 (頭殿長: 45mm、89mm、105mm、148mm、198mm) 選抜して作成し、画像処理ソフトで立体像の厚さを色で可視化した。作成した立体像を「厚さ、分枝の傾き、分枝の場所」の3点に着目し、左右差の有無を観察した。②DICOM-viewerソフトで血管断面を楕円形に近似し、長径と短径を測定した。長径と短径の相乗平均を血管の太さの指標とした (血管径とする) 。血管径の値から計算した左右比にt検定を行い、左右比が有意か血管毎に調べた。③長径と短径から血管断面の楕円率を計算した。楕円率から計算した左右比にt検定を行い、楕円率の左右比が有意か血管毎に調べた。【結果】①頭頸部•上肢の静脈として腕頭静脈、内頸静脈、鎖骨下静脈を観察した。腕頭静脈では厚さで左右差があった。具体的には頭殿長105mm以降の3例で左腕頭静脈にだけ途中で厚みが増している部分があった。残りの観点では左右差はなかった。内頸静脈、鎖骨下静脈ではいずれの観点でも左右差はなかった。②総頸動脈の血管径の左右比は有意に左が大きかった。具体的には総頚動脈は左が右を基準に1.15倍太かった (右の血管径中央値0.37 mm、左の血管径中央値0.53 mm、p=0.004) 。肺動脈は有意に右が太かった。すなわち、肺動脈は右が1.17倍太かった (右0.63mm、左0.47mm、p<0.001) 。③総腸骨静脈の断面の楕円率の左右比は有意に右が大きかった。具体的には総腸骨静脈の楕円率は右が1.79倍大きい (右の楕円率中央値0.66、左の楕円率中央値0.38、p<0.001) 。上肺静脈の楕円率の左右比は有意に右が大きかった。すなわち、上肺静脈の楕円率は右が1.71倍大きい (右0.65、左0.38、p<0.001) 。①②③それぞれについて、他の血管の結果および考察も報告する。【結論】頭殿長26.8mmから225mmの胎児のうち、5個体について主要な血管の立体像を作成および観察し、左右差の観点から定性的な特徴を示すことができた。また頭殿長26.8mmから225mmの胎児について主要な血管の左右差を定量的な観点から示すことができた。 24. Three-dimensional analysis of the area around the ankle joint in the human fetus ヒト胎児期における足関節周辺の三次元解析 松田幸樹 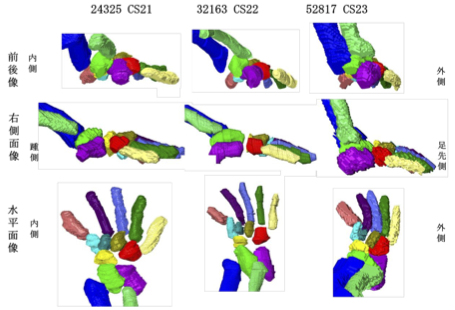 【背景】ヒトの足関節部の肢位は脛骨、腓骨、および足根骨のうち距骨、踵骨に より主に決定される。発生 5 週目末に脛骨、腓骨、足根骨が順に出現し、それぞ れの形態形成は、互いの位置関係に影響を与えながら進行する。胎児の骨形成や、 胎児期のある時期における下腿の骨の成長などについて論じた研究はあるが、胎 児期の成長に伴う足関節周辺の骨の位置関係の変化について論じた研究は少な く、また定量的な評価が十分になされた三次元的研究も乏しい。そこで本研究で は、ヒト胎児固定標本の T1 強調画像、位相 X 線 CT 画像から立体再構成像を取得 し、ヒト胎児期における足関節周辺の骨の形成、それらの位置関係の変化の追跡 を試みた。 【方法】対象は京都大学医学研究科附属先天異常標本解析センター保有 28 体(内 7T-MR 撮像 24 体、位相 X 線 CT 撮像 4 体)と、島根大学医学部解剖学教室保有の 9 体(T1 強調画像)である。これらを画像処理ソフトウェア Amira を用いて下肢骨 の立体再構成像を作成した。次に同ソフトウェアで、解剖学的ランドマーク、各 骨(大腿骨、脛骨、腓骨、足根骨)の重心、関節周辺の各骨の表面情報 を取得し た。その後 Amira で得たデータをもとに、MATLAB により長管骨の軸、関節表面(膝、 距腿、距腓、距踵関節)の疑似平面を作成して下肢骨の位置関係の解析、成長の 変化の定量的な観察を行った。 【結果】作成した足関節周辺の立体再構成像の観察を行った。冠状面において胚 子期後期から CRL34~50 mmの範囲の個体では足関節は内転の状態が観察された。ま た矢状面では胚子期後期から CRL34~43 mmの範囲では底屈の状態が観察された。ど ちらの肢位も CRL60 mm辺りでほぼ中間位をとった。また脛骨長軸の角度と合わせ て、抽出した関節面に法線ベクトルを定義し、それらの角度の CRL 増加に伴う変 化、関節同士の関係性、肢位の変化にどの関節が影響を与えているのかを検討した。CRL 増加に伴い、冠状面では脛骨長軸、距脛関節面法線ベクトル、距踵関節 面法線ベクトルの変化が見られた。矢状面では脛骨長軸、距脛関節面法線ベクト ルの変化が観察された。脛骨長軸と関節面法線ベクトルの関係性を評価したとこ ろ、冠状面では距脛関節面と距踵関節面が、矢状面では距脛関節面が脛骨長軸と 高い相関を示した。 【結論】足関節周辺の立体像を作成することにより、中足骨を含む足根骨から脛 骨、腓骨の長管骨までの領域における骨の位置関係について、胚子期後期の CS21 から胎児期初期までの経時的変化を提供することができた。また、肢位の変化や 関節面の方向を座標上で表現することにより、成長に伴う変化を定量的に示すこ とができた。将来的に、胚胎児期の下肢周辺における異常診断に有益な情報を与 えることが期待される。 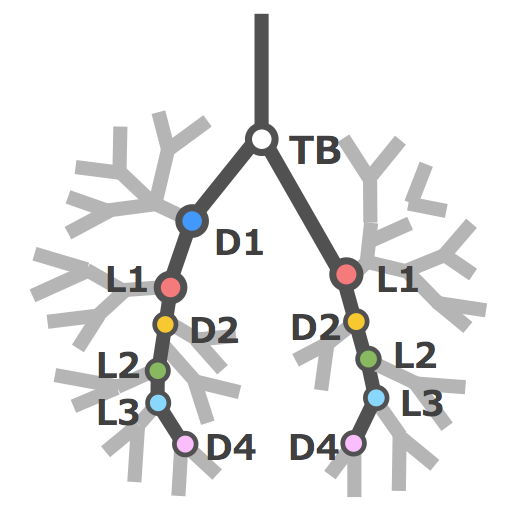
Fujii S, Muranaka T, Matsubayashi J, Yamada S, Yoneyama A, Takakuwa T. Bronchial tree of the human embryo: Examination based on a mammalian model. J Anatomy 2024, 244, 159-169 http://doi.org/10.1111/joa.13946 . The symmetry of right and left bronchi, proposed in a previous comparative anatomical study as the basic model of the mammalian bronchial tree, was examined to determine if it applied to the embryonic human bronchial tree. Imaging data of 41 human embryo specimens at Carnegie stage (CS) 16–23 (equivalent to 6–8 weeks after fertilization) belonging to the Kyoto collection were obtained using phase-contrast X-ray computed tomography. Three-dimensional bronchial trees were then reconstructed from these images. Bronchi branching from both main bronchi were labeled as dorsal, ventral, medial, or lateral systems based on the branching position with numbering starting cranially. The length from the tracheal bifurcation to the branching point of the labeled bronchus was measured, and the right-to-left ratio of the same labeled bronchus in both lungs was calculated. In both lungs, the human embryonic bronchial tree showed symmetry with an alternating pattern of dorsal and lateral systems up to segmental bronchus B9 as the basic shape, with a more peripheral variation. This pattern is similar to that described in adult human lungs. Bronchial length increased with the CS in all labeled bronchi, whereas the right-to-left ratio was constant at approximately 1.0. The data demonstrated that the prototype of the human adult bronchial branching structure is formed and maintained in the embryonic stage. The morphology and branching position of all lobar bronchi and B6, B8, B9, and the subsegmental bronchus of B10 may be genetically determined. On the other hand, no common structures between individual embryos were found in the peripheral branches after the subsegmental bronchus of B10, suggesting that branch formation in this region is influenced more by environmental factors than genetic factors. 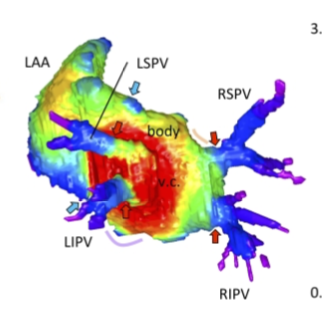 福井さんの修士論文がJ Anatomyに受諾されました。ヒト胎児期初期の左房の形成を肺静脈のとりこみと左心耳の形成を中心に解析しました。
Fukui N, Toru KanahashiT, MatsubayashiJ, ImaiH, YoneyamaA, OtaniH, YamadaS, Takakuwa T. Morphogenesis of the pulmonary vein and left atrial appendage in human embryos and early fetuses. J Anatomy 2024, 244, 142-158, in press, https://doi.org/10.1111/joa.13941 Abstract The left atrium wall has several origins, including the body, appendage, septum, atrial–ventricular canal, posterior wall, and venous component. Here, we describe the morphogenesis of left atrium based on high-resolution imaging (phase-contrast X-ray computed tomography and magnetic resonance imaging). Twenty-three human embryos and 19 fetuses were selected for this study. Three-dimensional cardiac images were reconstructed, and the pulmonary veins and left atrium, including the left atrial appendage, were evaluated morphologically and quantitatively. The positions of the pericardial reflections were used as landmarks for the border of the pericardial cavity. The common pulmonary vein was observed in three specimens at Carnegie stage 17–18. The pericardium was detected at the four pulmonary veins (left superior, left inferior, right superior, and right inferior pulmonary veins) at one specimen at Carnegie stage 18 and all larger specimens, except the four samples. Our results suggest that the position of the pericardial reflections was determined at two pulmonary veins (right and left pulmonary vein) and four pulmonary veins almost simultaneously when the dorsal mesocardial connection between the embryo and heart regressed. The magnetic resonance images and reconstructed heart cavity images confirmed that the left atrium folds were present at the junction between the body and venous component. Three-dimensional reconstruction showed that the four pulmonary veins entered the dorsal left atrium tangentially from the lateral to the medial direction. More specifically, the right pulmonary veins entered at a greater angle than the left pulmonary veins. The distance between the superior and inferior pulmonary veins was shorter than that between the left and right pulmonary veins. Three-dimensional reconstruction showed that the venous component increased proportionally with growth. No noticeable differences to discriminate between the right and left parts of the venous component emerged, while the junction between the venous component and body gradually became inconspicuous but was still recognizable by the end of the observed early fetal period. The left superior pulmonary vein had the smallest cross-sectional area and most flattened shape, whereas the other three were similar in area and shape. The left atrial appendage had a large volume in the center and extended to the periphery as a lobe-like structure. The left atrial appendage orifice increased in the area and tended to become flatter with growth. The whole left atrium volume^(1/3) increased almost proportionally with growth, parallel to the whole heart volume. This study provided a three-dimensional and quantitative description of the developmental process of left atrium, comprising the venous component and left atrial appendage formation, from the late embryonic to the early fetal stages.  第99回日本解剖学会学術集会近畿支部で発表しました(11/18、京都) 松田幸樹、松林潤、金橋徹、山田重人、大谷浩、高桑徹也、ヒト胎児期における足関節周辺の三次元解析 金橋徹、松林潤、今井宏彦、山田重人、大谷浩、高桑徹也、ヒト胎児骨盤の性差の検討  第17回日本臨床検査学教育学会学術大会 (2023.8.23-24, 天理)で発表しました。 八田桃佳、金橋徹、今井宏彦、山田重人、高桑 徹也、拡散テンソル画像を用いた水晶体線維細胞の配向性の検討 *八田さんが 優秀発表賞(大学院)を受賞しました。おめでとうございます!! 中井尚一、松林潤、金橋徹、今井宏彦、山田重人、大谷浩、高桑徹也、ヒト胎児期初期から中期の血管径変化より紐解く循環動態の検討 金橋 徹、伊吹謙太郎、山田 稔、榛葉旭恒、高桑徹也、寄生虫顕微鏡スライド標本のデジタル化に向けた取り組み    第63回日本先天異常学会で発表しました (2023.7.28-30、つくば) 石田七彩、植田優生、松林潤、金橋徹、今井宏彦、山田重人、高桑徹也、生理的臍帯ヘルニア期のヒト中腸ループと腸間膜の経時的構造変化 岩佐結生、金橋徹、松林潤、今井宏彦、大谷 浩、高桑徹也、腹直筋の腱画形成過程 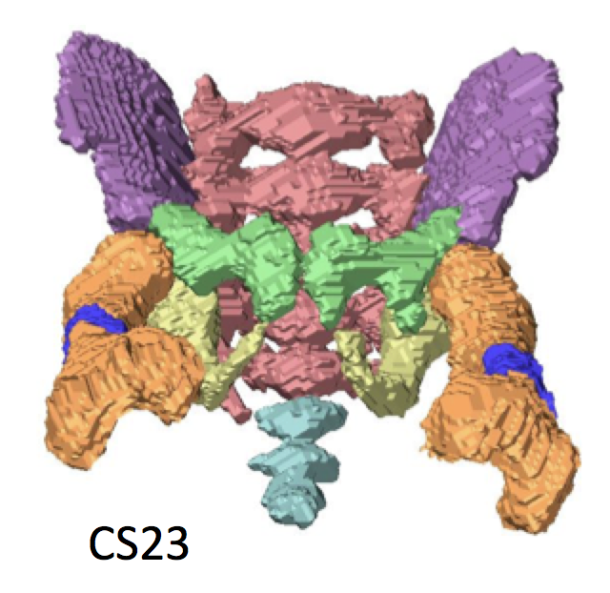 胚子期の股関節の肢位についての論文がPLoS Oneに掲載されました。熊野くんが卒業研究で行ったデータに、胚子期の標本のデータを追加し解析しました。大腿骨(股関節)の肢位を正確に測定し、下肢の発達の時間軸を確立することを目的とした論文です。
60. Takakuwa T, Saizonou MA, Fujii S, Kumano Y, Ishikawa A, Aoyama T, Imai H, Yamada S, Kanahashi T. Femoral posture during embryonic and early fetal development: An analysis using landmarks on the cartilaginous skeletons of ex vivo human specimens. PLOS one, 2023, 18(5): e0285190. https://doi.org/10.1371/journal.pone.0285190. 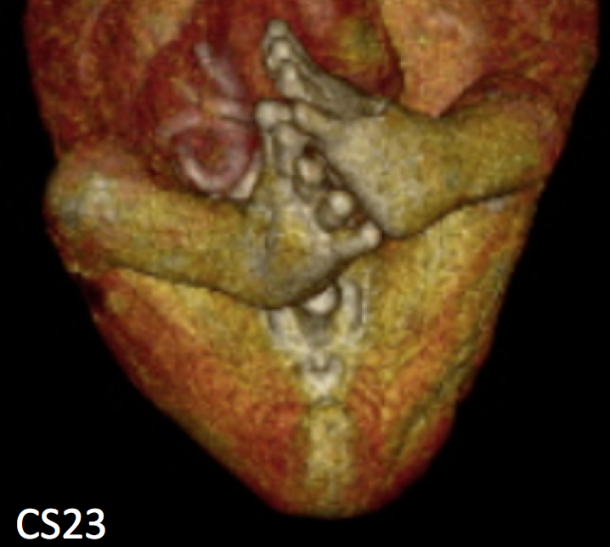 AbstractThe pre-axial border medially moves between the fetal and early postnatal periods, and the foot sole can be placed on the ground. Nonetheless, the precise timeline when this posture is achieved remains poorly understood. The hip joint is the most freely movable joint in the lower limbs and largely determines the lower-limb posture. The present study aimed to establish a timeline of lower-limb development using a precise measurement of femoral posture. Magnetic resonance images of 157 human embryonic samples (Carnegie stages [CS] 19–23) and 18 fetal samples (crown rump length: 37.2–225 mm) from the Kyoto Collection were obtained. Three-dimensional coordinates of eight selected landmarks in the lower limbs and pelvis were used to calculate the femoral posture. Hip flexion was approximately 14° at CS19 and gradually increased to approximately 65° at CS23; the flexion angle ranged from 90° to 120° during the fetal period. Hip joint abduction was approximately 78° at CS19 and gradually decreased to approximately 27° at CS23; the average angle was approximately 13° during the fetal period. Lateral rotation was greater than 90° at CS19 and CS21 and decreased to approximately 65° at CS23; the average angle was approximately 43° during the fetal period. During the embryonic period, three posture parameters (namely, flexion, abduction, and lateral rotation of the hip) were linearly correlated with each other, suggesting that the femoral posture at each stage was three-dimensionally constant and exhibited gradual and smooth change according to growth. During the fetal period, these parameters varied among individuals, with no obvious trend. Our study has merits in that lengths and angles were measured on anatomical landmarks of the skeletal system. Our obtained data may contribute to understanding development from anatomical aspects and provide valuable insights for clinical application. |
||||