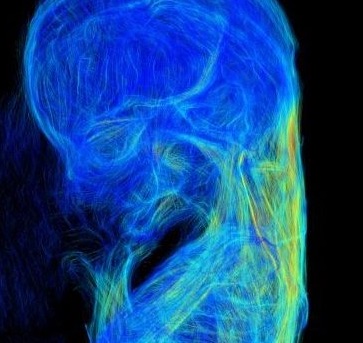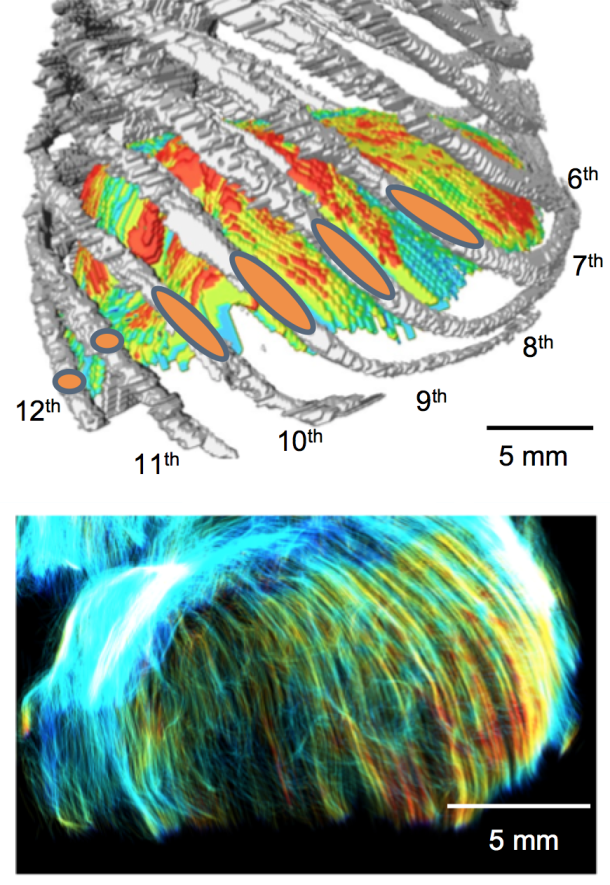
金橋先生の横隔膜形成についての論文がJ. Anat.に掲載されました。また雑誌表紙に採用されました。
この研究は、胚子期後期から胎児期初期のヒト横隔膜の形態形成および線維構造の質的評価をすることを目的とし、従来使用しているT1強調像に加えて、DTIを用いた高解像度MRI画像を導入して解析しました。
- 横隔膜はCS20で完全に閉鎖した
- CRLが46mm以上のサンプルでは、胸骨、肋骨、腰部、および食道裂孔を囲む領域が肥厚したが、横隔膜の中心や左右の横隔膜ドーム頂部の厚さは変化しない。
- 肋骨と腰部のすべての線維は、大静脈開口部と食道裂孔周囲を除いて、左右の半横隔膜ドームに向かって走行した
Kanahashi T, Imai H, Otani H, Yamada S, Yoneyama A, Takakuwa T. Three-dimensional morphogenesis of the human diaphragm during the late embryonic and early fetal period: Analysis using T1-weighted and diffusion tensor imaging. J Anat. 2023, 242, 174-190, DOI: 10.1111/joa.13760
Abstract

A precise understanding of human diaphragm development is essential in fetal medicine. To our knowledge, no previous study has attempted a three-dimensional (3-D) analysis and evaluation of diaphragmatic morphogenesis and development from the embryonic to the early fetal period. This study aimed to evaluate the morphogenesis and fibrous architecture of the developing human diaphragm during the late embryonic and early fetal periods. Fifty-seven human embryos and fetuses (crown-rump length [CRL] = 8–88 mm) preserved at the Congenital Anomaly Research Center of Kyoto University and Shimane University were analyzed. 3-D morphogenesis and fiber orientation of the diaphragm were assessed using phase-contrast X-ray computed tomography, T1-weighted magnetic resonance imaging (T1W MRI), and diffusion tensor imaging (DTI). T1W MR images and DTI scans were obtained using a 7-T MR system. The diaphragm was completely closed at Carnegie stage (CS) 20 and gradually developed a dome-like shape. The diaphragm was already in contact with the heart and liver ventrally in the earliest CS16 specimen observed, and the adrenal glands dorsally at CS19 or later. In the fetal period, the diaphragm contacted the gastric fundus in samples with a CRL ≥41 mm, and the spleen in samples with a CRL ≥70 mm. The relative position of the diaphragm with reference to the vertebrae changed rapidly from CS16 to CS19. The most cranial point of the diaphragm was located between the 4th and 8th thoracic vertebrae, regardless of fetal growth, in samples with a CRL ≥16 mm. Diaphragmatic thickness was nearly uniform (0.15–0.2 mm) across samples with a CRL of 8 mm to 41 mm. The sternal, costal, lumbar parts, and the area surrounding the esophageal hiatus thickened with growth in samples with a CRL ≥46 mm. The thickness at the center of the diaphragm and the left and right hemidiaphragmatic domes did not increase with growth. Tractography showed that the fiber orientation of the sternal, costal, and lumbar parts became more distinct as growth progressed in CS19 or later. All fibers in the costal and lumbar regions ran toward the left and right hemidiaphragmatic domes, except for those running to the caval opening and esophageal hiatus. The fiber orientation patterns from the right and left crura surrounding the esophageal hiatus were classified into three types. Distinct fiber directions between the costal and sternal, and between the costal and lumbar diaphragmatic parts were observable in samples with a CRL ≥46 mm. Anterior costal and sternal fibers ran toward the center. Fiber tracts around the center and the left and right hemidiaphragmatic domes; between the costal and lumbar orientations; and between the costal and sternal orientations showed a tendency for decreasing fractional anisotropy values with fetal growth, and showed less density than other areas. In conclusion, we used 3-D thickness assessment and DTI tractography to identify qualitative changes in the muscular and tendonous regions of the diaphragm during the embryonic and early fetal periods. This study provides information on normal human diaphragm development for the progression of fetal medicine and furthering the understanding of congenital anomalies.